Grant title: Understanding the role of metabolic health in lifestyle related cancers through proteomics
Institution: International Agency for Research on Cancer
Grant awarded: September 2025
Receiving the World Cancer Research Fund grant is a tremendous honour that will allow us to investigate the critical role of metabolic health in cancer development through cutting-edge proteomics. The ProMetLife project will help uncover the biological mechanisms linking metabolic syndrome to lifestyle-related cancers, which is essential for developing new prevention strategies – Dr Komodo Matta
Background
Almost half of all cancer cases can be attributed to unhealthy lifestyle behaviours and can thus be prevented. Poor lifestyle habits lead to metabolic dysfunction (measured by excess belly fat, high blood pressure, high bad cholesterol and low good cholesterol, and high blood sugar), which has been linked to developing several different cancers. It is not fully known how metabolic dysfunction leads to cancer, but the answer may lie in proteins in the blood.
Proteins are the building blocks of life and are involved in every biological process. Their levels in the blood may be impacted by metabolic dysfunction. New data on blood protein levels are now available in large population studies, allowing us to explore the potential role of proteins in the relationship between metabolic dysfunction and cancer risk.
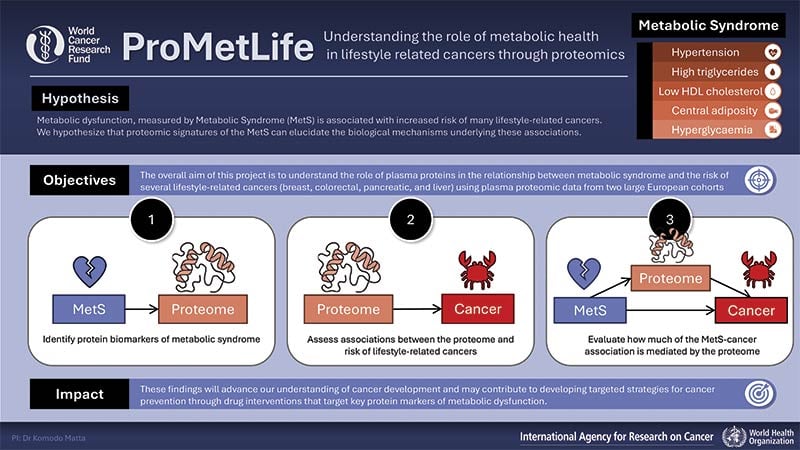
Hypothesis and objectives
In this study, we hope to understand how metabolic dysfunction leads to the development of lifestyle-related cancers. We hypothesise that metabolic dysfunction can be measured using protein levels in the blood. Subsequently, we hypothesise that these metabolic-related proteins are linked to cancer.
To test these hypotheses, we first aim to relate metabolic dysfunction to proteomic markers (individual and combinations of protein levels as well as to protein profiles). These protein levels will act as a signature of metabolic dysfunction. Then, we will assess the relationship between these proteins and cancer risk. If the proteins are linked to cancer risk, we will find out how much of the relationship between metabolic dysfunction and cancer can be explained through the blood proteins.
By identifying proteins related to metabolic dysfunction and examining their role in cancer risk, we aim to better understand the biological processes linking poor metabolic health to the development of lifestyle-related cancers including breast, colorectal, pancreatic and liver.
How it will be done
We will use data collected from participants of 2 large studies: UK Biobank and European Prospective Investigation into Cancer and Nutrition (EPIC). In both studies, participants provided detailed information on sociodemographic characteristics, body measurements and lifestyle factors through questionnaires. Participants were followed for around 20 years and consented to have data collected on clinical information, including cancer diagnoses and deaths. From UK Biobank, we have data on 3,000 blood proteins from 54,000 participants. From EPIC, we have data on more than 6,400 blood proteins in 18,000 people. Using appropriate statistical tools, we will assess the relationship between different measures of metabolic dysfunction and blood protein levels, and subsequently evaluate whether or to what extent these proteins impact cancer risk.
Created by Dr Komodo Matta, International Agency for Research on Cancer
Potential impact
This study will be the first to link proteomic markers of metabolic dysfunction with multiple cancers. By identifying protein profiles linked to metabolic dysfunction that are associated to cancer risk, we will elucidate the biological pathways by which metabolic dysfunction leads to cancer. These findings will advance our understanding of the development of cancer and may contribute to developing strategies for cancer prevention through drug interventions that target key protein markers.
